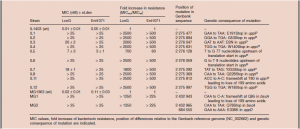
The bacterial strains are resistant to the two peptide bacteriocins (antimicrobial peptides) lactococcin G and enterocin 1071 and most likely to lactococcin Q. It has been shown that resistance to high lactococcin G concentrations occurred due to mutations in or near the gene uppP (bacA), encoding an undecaprenyl pyrophosphate phosphatase; a membrane protein involved in peptidoglycan synthesis.
The mutant strains were also assayed against the lactococcin G-like two-peptide bacteriocin enterocin 1071, which has 57% sequence identity with lactococcin G. Overall, the degree of resistance to enterocin 1071 was similar to what was observed for lactococcin G. These mutants (JK-1 to -6 and JK-8 to 11) were resistant to the highest enterocin 1071 concentration tested (except the strain IL5). These results suggest that the same genetic determinant(s) are involved in the sensitivity to lactococcin G and the related bacteriocin enterocin 1071.